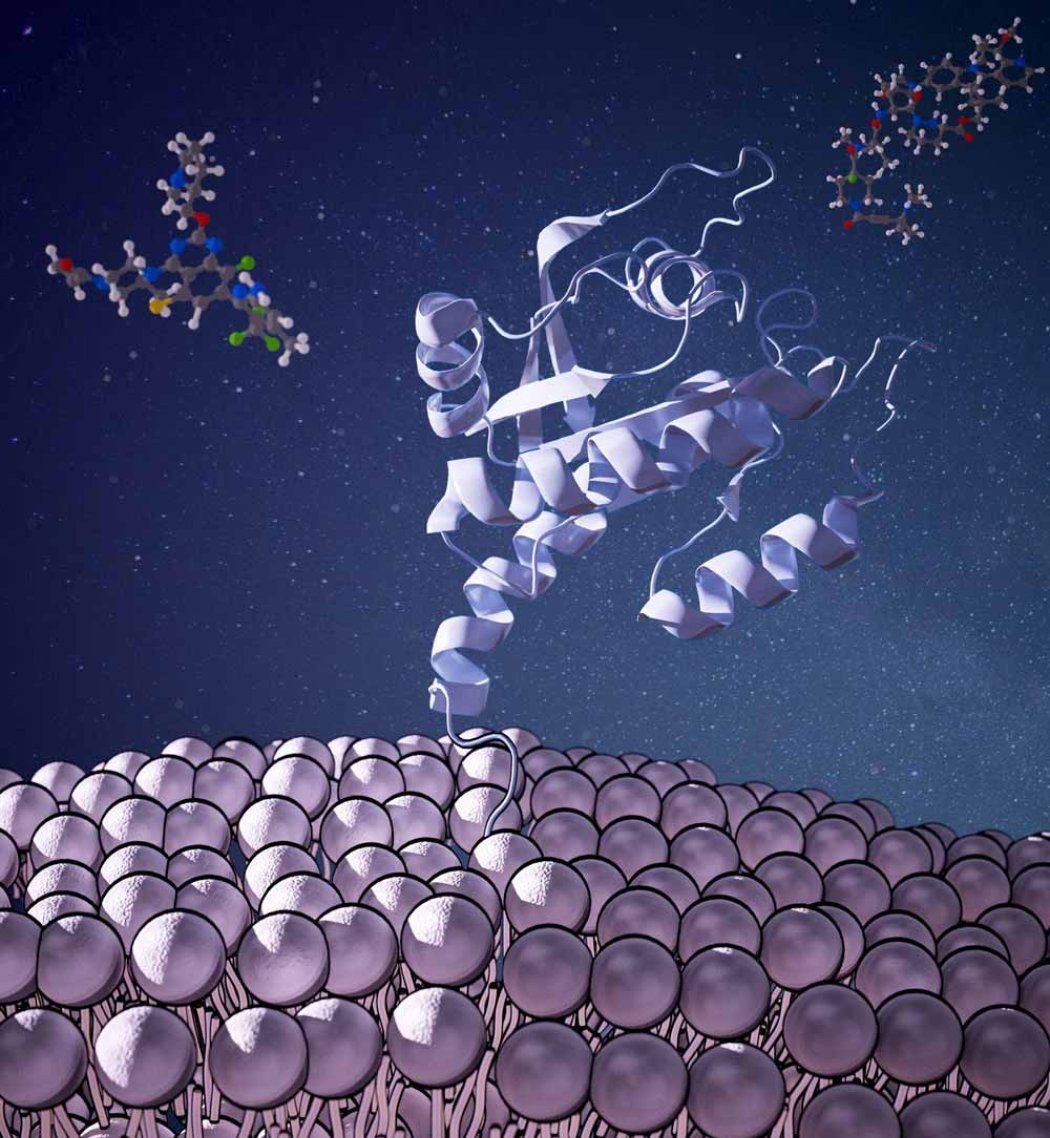
UCSF scientists have discovered how to target a large family of molecular switches called GTPases that are involved in myriad diseases, from Parkinson’s to cancer, and have long been thought to be “undruggable.”
Because of their slippery exterior, this class of more than 100 enzymes has remained largely out of reach of modern drug discovery, with the exception of the notorious cancer-causing GTPase called K-Ras.
In 2013, Kevan Shokat, PhD, and his colleagues discovered a “pocket” where drugs could bind to K-Ras, a famous GTPase that’s responsible for up to 30% of all cancer cases.
Since then, nearly a dozen drugs have been developed targeting mutations to K-Ras, but the other GTPases remained untouchable.
On a hunch, the team used a K-Ras mutation as a tool to try to make a handful of these molecular switches more receptive to drugs, and then tested a dozen drugs that target K-Ras. The approach revealed new drug binding sites that could not have been predicted by the computational drug discovery tools that drive most new drug development.
The findings, published on Sept. 9 in Cell, create a long-awaited opportunity to develop therapies for the many diverse diseases that arise from GTPase dysfunction.
“We’ve known about the GTPases for decades but have lacked any way to reliably drug them,” said Shokat, UCSF professor in the Department of Cellular and Molecular Pharmacology and senior author of the paper. “This really puts all those GTPases on the map for drug discovery, so it’s possible to target them when they’re associated with disease.”

The hook that caught the mouthless fish
Our cells depend on networks of GTPases, which oversee everything from the movement of molecules to cell growth and division. When something goes wrong among these switches, disease can follow.
In the present work, Shokat’s team, led by UCSF postdoctoral scholar and first author Johannes Morstein, PhD, engineered one of the cancer-causing K-Ras mutations, known as G12C, into a representative group of GTPases.
They guessed that G12C, which places a chemical “hook” onto a protein, could help them fish out which K-Ras G12C drugs might bind to other GTPases, which have only subtle similarities to K-Ras itself.
The laboratory experiments turned up gold: with the help of G12C, some of the K-Ras drugs bound to the otherwise featureless GTPases. When G12C was removed, those drugs still bound to the GTPase.
Freezing the switch between on and off
The approach, dubbed chemical genetics, exploited the flexibility of the GTPases, enabling the drugs to nudge open a pocket in the protein where it could lodge. This pocket had evaded previous efforts to predict, computationally, where drugs might bind.
“Since these GTPases switch between ‘on’ and ‘off’ states, the pocket is not usually visible, certainly not to the standard software used for drug discovery,” Shokat said. “Instead, the drug binds to an intermediate state, freezing the GTPases and inactivating them.”
The researchers are sharing their methods openly in the hopes that others will use them to drug their GTPase of interest, whether it’s a Rab GTPase, which is implicated in Alzheimer’s, or a Rac GTPase, which plays a role in breast cancer. Among the hundreds of GTPases, there’s rich potential to make progress for patients.
“In the case of these enzymes, it was critical for us to first test our ideas experimentally in the laboratory, to actually see what worked,” Morstein said. “We’re hopeful it can really accelerate drug discovery.”
Other authors: Critical contributions were made by researchers at Lawrence Livermore National Lab and the National Cancer Institute’s RAS initiative. Other UCSF authors are Victoria Bowcut, Micah Fernando, Lawrence Zhu, Keelan Z Guiley, Matthew Peacock, Zhi Lin, Katrine A. Taran, and Benjamin J Huang. For all authors, see the paper.
Funding and disclosures: The work was supported by in part by federal grants (K99CA277358, 5R01CA244550, NSERC-2020-04241, 75N91019D00024). Several authors are inventors on patents owned by UCSF covering GTPase-targeting small molecules. For all funding and disclosures, see the paper.