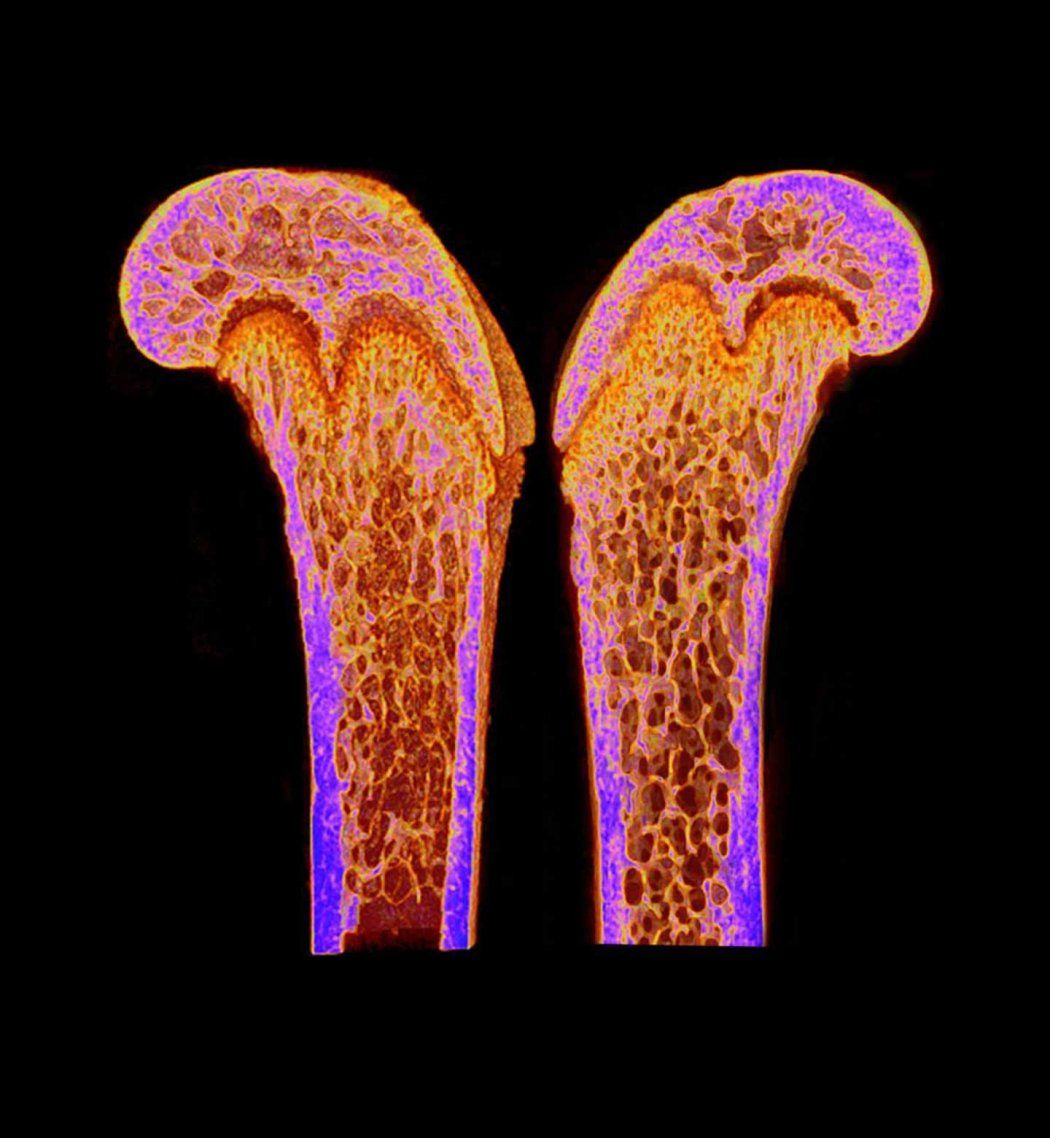
A newly discovered hormone that keeps the bones of breastfeeding women strong could also help bone fractures heal and treat osteoporosis in the broader population. Researchers at UC San Francisco and UC Davis showed that in mice, the hormone known as Maternal Brain Hormone (CCN3) increases bone density and strength.
Their results, published July 10 in Nature, solve a long-standing puzzle about how women’s bones remain relatively robust during breastfeeding, even as calcium is stripped from bones to support milk production.
“One of the remarkable things about these findings is that if we hadn’t been studying female mice, which unfortunately is the norm in biomedical research, then we could have completely missed out on this finding,” said Holly Ingraham, PhD, the senior author of the new paper and a professor cellular molecular pharmacology at UCSF. “It underscores just how important it is to look at both male and female animals across the lifespan to get a full understanding of biology.”
More than 200 million people worldwide suffer from osteoporosis, a severe weakening of the bones that can cause frequent fractures. Women are at particularly high risk of osteoporosis after menopause because of declining levels of the sex hormone estrogen, which normally promotes bone formation. Estrogen levels are also low during breastfeeding, yet osteoporosis and bone fractures are much rarer during this time, suggesting that something other than estrogen promotes bone growth.
A hormone that is only produced during lactation
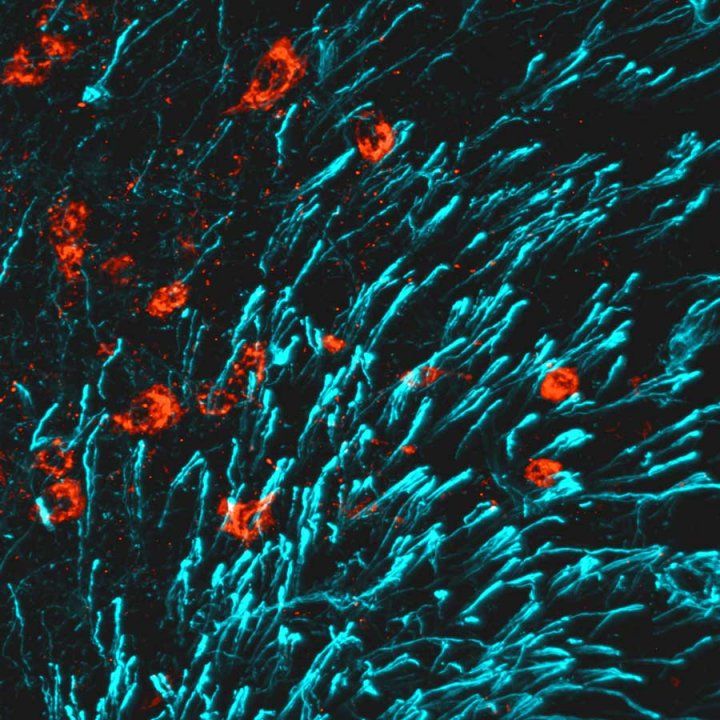
Ingraham’s lab previously discovered that in female mice, but not male mice, blocking a particular estrogen receptor found in select neurons in a small area of the brain led to huge increases in bone mass. They suspected that a hormone in the blood was responsible for the super-strong bones but, at the time, could not find it – a quest that was further protracted during the worldwide pandemic.
In the new work, Ingraham and collaborators carried out an exhaustive search for this bone-building hormone and finally pinpointed CCN3 as the factor responsible in mutant females. Initially, the team was surprised by this result, as CCN3 did not fit the typical profile of a secreted hormone from neurons.
Their doubts vanished after they found CCN3 in the same brain region in lactating female mice. Without the production of CCN3 in these select neurons, lactating female mice rapidly lost bone, and their babies began to lose weight, confirming the importance of the hormone in maintaining bone health during lactation. Based on this discovery, they now refer to CCN3 as Maternal Brain Hormone (MBH).
Hormone strengthens bones in young, old, male and female mice
When strategies to increase circulating CCN3 were implemented in young adult and older female or male mice, their bone mass and strength increased dramatically over the course of weeks. In some female mice who lacked all estrogen or were very old, CCN3 was able to more than double bone mass.
When Ingraham’s scientific collaborator, Thomas Ambrosi, PhD, an assistant professor of orthopaedic surgery at UC Davis, tested these bones, he was surprised by their strength.
“There are some situations where highly mineralized bones are not better; they can be weaker and actually break more easily,” he explained. “But when we tested these bones, they turned out to be much stronger than usual.”
Ambrosi looked closely at the stem cells within the bones that are responsible for generating new bone and found that when these cells were exposed to CCN3, they were much more prone to generate new bone cells.
A hydrogel patch heals fractures in mice
To test the ability of the hormone to assist in bone healing, the researchers created a hydrogel patch that could be applied directly to the site of a bone fracture, where it would slowly release CCN3 for two weeks. In elderly mice, bone fractures don’t usually heal well. However, the CCN3 patch spurred the formation of new bone at the site of the fracture, contributing to youthful healing of the fracture.
“We’ve never been able to achieve this kind of mineralization and healing outcome with any other strategy,” Ambrosi said. “We’re really excited to follow it up and potentially apply CCN3 in the context of other problems, such as regrowing cartilage.”
The researchers plan to carry out future studies on the molecular mechanisms of CCN3, its levels in breastfeeding women, as well as the potential of the hormone to treat a variety of bone conditions.
Muriel Babey, MD, a co-first author and mentored physician-scientist in the Division of Endocrinology at UCSF, is keen to begin asking how CCN3 impacts bone metabolism in clinically relevant disease settings. Partnering with the UCSF Catalyst program, William Krause, PhD, a senior scientist and co-lead on this project will begin translating these new results.
“Bone loss happens not only in post-menopausal women but often occurs in breast cancer survivors that take certain hormone blockers; in younger, highly trained elite female athletes; and in older men whose relative survival rate is poorer than women after a hip fracture,” Ingraham said. “It would be incredibly exciting if CCN3 could increase bone mass in all these scenarios.”

Authors: Other UCSF authors include Candice B. Herber, Zsofia Torok, Joni Nikkanen, Ruben Rodriquez, Saul Villeda and Fernanda Castro-Navarro. Other UC Davis authors include Kun Chen, Erika E. Wheeler, J. Kent Leach, and Nancy E. Lane. Please see the paper for a complete author list.
Funding: This work was supported by the National Institutes of Health (R01DK121657-S1, NIA-1K01AG065916, 5K12GM081266, K99DK129763, AG066963, R01DK132073, R01AG067740, R01AG070647, R01AG062331, R01DK121657), a Stanford Pilot Award and a Senior Scholar Award (GCRLE0320).
Disclosures: None.