How Neurons Build a 3-D Vascular Structure to Keep the Retina Healthy
Understanding how intricate networks of blood vessels in the eye and brain are formed could inspire new treatments for conditions like diabetic retinopathy and stroke.
Scientists have known for years that a lattice of blood vessels nourishes cells in the retina that allow us to see – but it’s been a mystery how the intricate structure is created.
Now, researchers at UC San Francisco have found a new type of neuron that guides its formation.
The discovery, described in the May 23, 2024, issue of Cell, could one day lead to new therapies for diseases that are related to impaired blood flow in the eyes and brain.
“This is the first time anyone has seen retinal neurons using direct contact with blood vessels as a way of guiding them to form these precise 3-D lattices,” said Xin Duan, PhD, an associate professor of ophthalmology and senior author of the study. “This brings us closer to the possibility of repairing them when they’re damaged or rerouting them when they weren’t built right in the first place.”
A protein that senses the presence of nearby cells
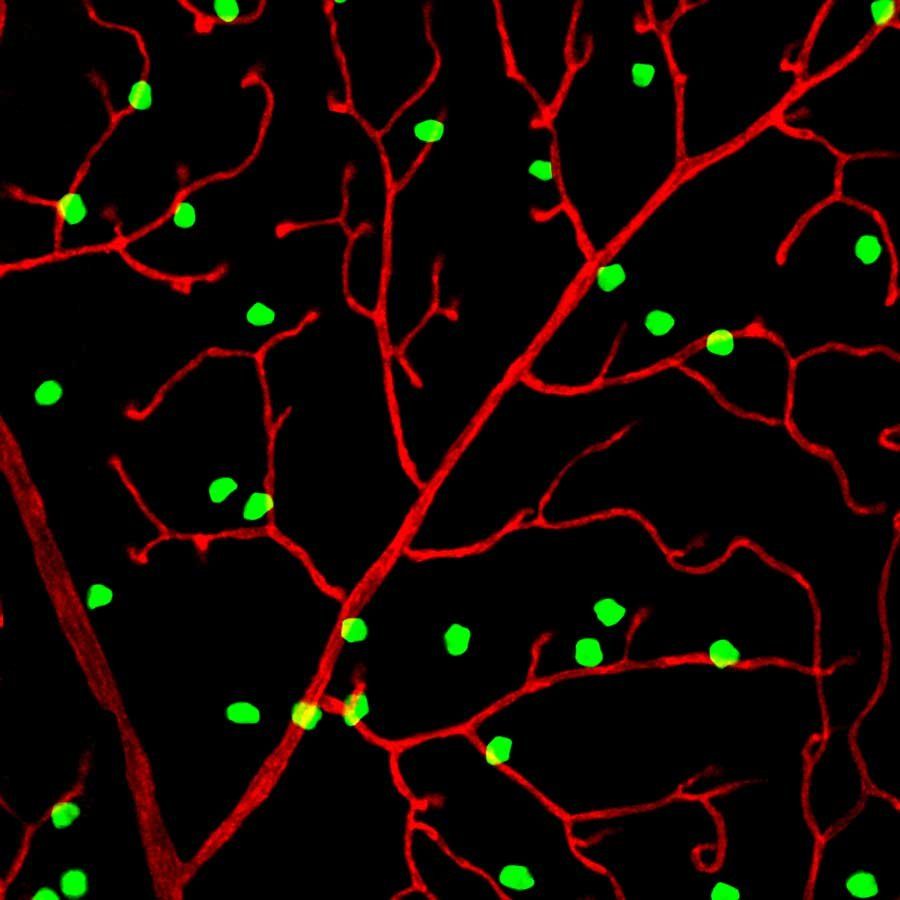
The researchers worked with newborn mice, whose eyes still need several weeks to develop fully. Kenichi Toma, PhD, labeled the retinal neurons closest to the blood vessels with a protein that glows green under ultraviolet light so he could observe the lattice as it was forming.
The team then identified a subset of neurons, called perivascular neurons, which contact and then surround growing blood vessels, directing them to form the lattice. These perivascular neurons produce a protein called PIEZO2 that enables them to sense when they are touching another cell.
Perivascular neurons in mice that were unable to produce PIEZO2 could not maintain contact with blood vessels, and they grew in a tangled, disorganized way that disrupted blood flow.
Starved for oxygen, the surrounding nerve cells degraded, and the mice were more vulnerable to stroke-like injuries.
Duan found that these neurons guide the formation of a similar network of blood vessels in the cerebellum, a part of the brain that is involved in coordination, language and sense perception.
“The fact that we see this same pattern repeated in the brain means that damage to this lattice might have a role in multiple neurodegenerative diseases,” Toma said.
The team collaborated with developmental biologist Arnold Kriegstein, MD, PhD, to confirm that perivascular retinal neurons also exist in humans.
3-D view shows how the lattice forms
Most research to date on the connection between the vascular and nervous systems has been limited by technology that only allows scientists to take two-dimensional pictures.
But Duan and Toma benefited from a new technique, using multiphoton microscopy, that Tyson Kim, MD, PhD, an assistant professor of ophthalmology, had developed to make 3-D images of retinal blood networks without disturbing the eye.
Kim helped Toma create revolving movies that captured the lattice from every angle and showed how it broke down in the absence of PIEZO2.
3-D video of the lattice of blood vessels that nourish cells in the retina. The colors show the three layers of the lattice. Blue vessels are closest to the surface, green vessels span the depth of the retina, and red is the layer deepest in the eye. Credit: Kenichi Toma, PhD
“We had been wanting to collaborate for some time, and this was the perfect opportunity,” Kim said. “It was really a confluence of what we’re each passionate about.”
A new way to protect neurons
The discoveries could inspire new ways of treating neurodegenerative diseases by ensuring that neurons, which demand a lot of energy, maintain a healthy blood supply.
“There are lots of people trying to understand the ways we can grow neurons,” Duan said. “But how in the world do we grow the intricate networks of blood vessels required to support them? That’s the question we’re trying to answer.”
Authors: Additional UCSF authors include Mengya Zhao, Shaobo Zhang, Fei Wang, Hannah K. Graham, Wenhao H. Shang, Nicole Y. Tsai, Guiying Hong, Tyson N. Kim, and Arnold Kriegstein. Other authors: Jun Zou and Xin Ye of Genentech, Liping Liu, Shweta Modgil, Yang Hu, and Joyce Liao of Stanford, Zhishun Cai and Ruobing Zhang of Suzhou Institute of Biomedical Engineering and Technology, and Jakob Korbelin of University Medical Center Hamburg-Eppendorf.
Funding: This study was supported by grants from the National Eye Institute (F30EY033201, K08EY033030, and R01EY030138), NINDS (R35NS097305) and the Glaucoma Research Foundation.