Key Pancreatic Cancer Defect Identified by UCSF Researchers
Pancreatic cancer, compared to other types of leading cancer killers, is detected later and does not respond well to either new or established drugs.
Now a new UC San Francisco study has shed light on the origins of one type of deadly pancreatic cancer.
In a study published online on Feb. 23 in the journal Nature Cell Biology, researchers led by Matthias Hebrok, PhD, head of the UCSF Diabetes Center, reported the identification of a key gene that normally holds tumor development at bay but, when damaged by mutation, allows pancreatic tumors to develop.
Absent advances that could lead to earlier diagnosis and better treatment, only about 6 percent of those in the United States who are diagnosed with pancreatic cancer will be alive five years from now, according to a National Cancer Institute prediction. In the United States, pancreatic cancer receives little press but now kills nearly as many as breast cancer. The American Cancer Society estimates that 40,430 will die of breast cancer in 2014, while 39,590 will die of pancreatic cancer.
In their latest study, the UCSF researchers used a new mouse model they bred especially to explore a mutated gene, called Brg1, or SMARCA4 in humans. They found that loss of the protein encoded by the gene – a result of genetic mutation – helps to trigger development of a type of pancreatic cancer thought to arise from cells lining the digestive-enzyme-secreting ducts of the pancreas.
Brg1 Mutations in Pancreatic Cancer
Rapid advances in scientists’ ability to unravel genomes of both tumors and normal tissues have led to the discovery of many genes that are abnormal in certain cancers. However, not all of these abnormal mutations in cancer drive tumor growth, and the biological importance of many of these mutated genes awaits study in cells grown in the lab or in animal models.

Matthias Hebrok, PhD
Previous studies of pancreatic cancer often have focused on the role of a mutated protein called K-ras. K-ras is implicated in almost all cases of pancreatic cancer and is common in many other cancers. K-Ras has so far been notoriously difficult to target with new drugs, although UCSF researchers led by Kevan Shokat, PhD, recently identified a promising K-ras targeting strategy.
Hebrok’s team is exploring how other mutations – including Brg1 mutations – influence the development of pancreatic cancer in cells with mutated K-ras.
According to Hebrok, who studies both the normal and abnormal development of the pancreas, improved mouse models can help researchers sort out the different ways cancers can form within a single organ type. For instance, he and others have found evidence that pancreatic tumors that form from duct cells arise differently from those that arise from another type of pancreatic cell, called the acinar cell. The cancer type that arises from pancreatic duct cells has been less deadly when detected early.
“We are investigating preliminary evidence that pancreatic duct cells must lose their specialized characteristics, a process called de-differentiation, before they can become cancerous,” Hebrok said. “A K-ras mutation in a pancreatic duct cell cannot drive this process in a vacuum, but elimination of normally functioning Brg1 following K-ras mutation may drive tumor development.”
Targeting Brg1 Genome Readers
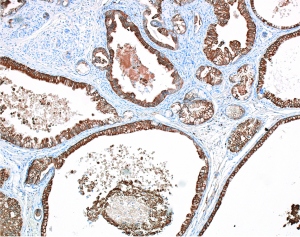
In cells that originate from pancreatic ducts, UCSF researchers led by Matthais Hebrok, PhD, have found that mutations to the genes Brg1 and K-ras can lead to the formation of cysts that may become deadly pancreatic cancers.
New models of pancreatic cancer, such as the one developed by Hebrok’s group, are valuable in pre-clinical studies of how new drugs might work in different subtypes of pancreatic cancer, and might lead to progress in developing treatments that are better tailored to individual patients, noted UCSF oncologist Eric Collisson, MD, who collaborates with Hebrok but who did not participate in the Nature Cell Biology study.
“The study of Brg1 highlights a whole new family of genes called chromatin-remodeling genes,” Collisson said.
Cancer researchers previously identified scores of proteins in cancer that become over-active to drive tumor growth, and others that hold growth in check but that are lost in cancer. Brg1 acts at a higher level.
Within the cell’s chromosomes, genes normally are packaged and folded together with proteins into a material called chromatin. The Brg1 protein teams up with other proteins to cover or uncover genes within chromatin, and this accessibility helps determine whether genes may be turned on or off.
“Brg1 helps a cell decide which genes are translated into protein,” Collisson said. “We are hoping that new classes of drugs that target these genome readers might one day prove useful in fighting pancreatic cancer.”
Additional study authors are Guido von Figura, Akihisa Fukuda, Nilotpal Roy, Muluye Liku, John Morris IV, Holger Russ and Grace Kim from UCSF; Matthew Firpo and Sean Mulvihill from the University of Utah; David Dawson from UCLA; Jorge Ferrer from Imperial College London; and William F.Mueller, Anke Busch, Klemens Hertel from UC Irvine.
Research funders included the U.S. National Institutes of Health, the Deutsche Forschungsgemeinschaft, the Klein Family Foundation, the Japan Society for the Promotion of Science, the U.S. National Pancreas Foundation, the Kato Memorial Biosciences Foundation and the California Institute for Regenerative Medicine.
UCSF is a leading university dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. It includes top-ranked graduate schools of dentistry, medicine, nursing and pharmacy, a graduate division with nationally renowned programs in basic biomedical, translational and population sciences, as well as a preeminent biomedical research enterprise and two top-ranked hospitals, UCSF Medical Center and UCSF Benioff Children’s Hospital.
