Behind the Scenes, a Race for Lifesaving Treatment
Infectious disease pharmacist Katherine Yang navigated the uncharted emergency-drug system – and helped save lives.

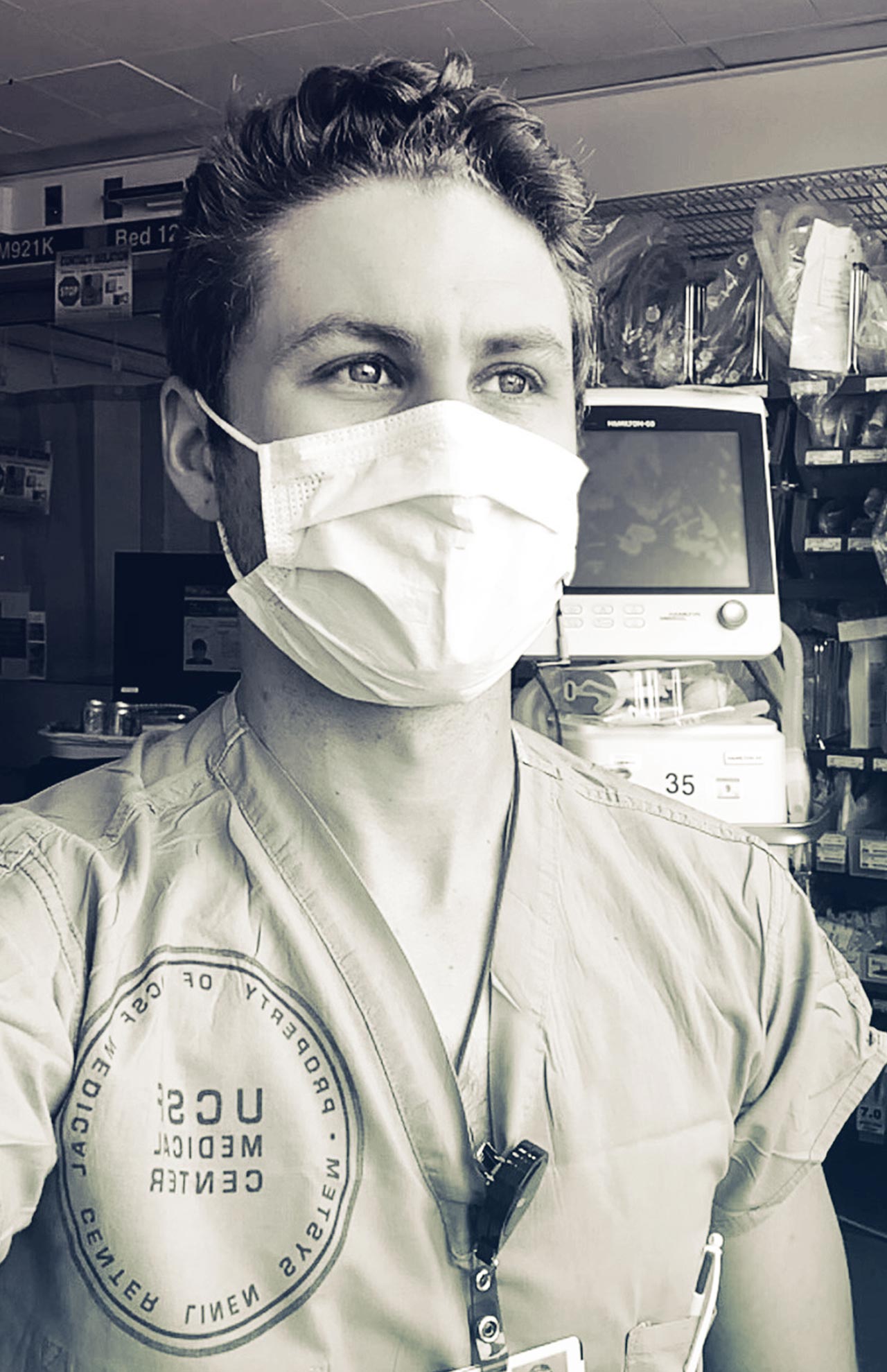
Katherine Yang, captured via FaceTime in the inpatient pharmacy at UCSF Helen Diller Medical Center’s Parnassus campus on June 12 at 10:30 a.m. by photographer Steve Babuljak
UCSF’s infectious disease consult service includes specially trained pharmacists with expertise in drug dosing, drug interactions, and drug approvals. A common maxim in medicine is, “If you hear hoofbeats, think horses, not zebras.” “We treat the zebras,” says Katherine Yang, PharmD ’98, MPH. “We don’t treat things like ear infections; we see complex infections or unusual pathogens, including the coronavirus.”
Yang, a clinical professor in the School of Pharmacy, recounts in her own words how her team mobilized to get a new drug approved for emergency COVID-19 treatment during the pandemic’s rise in San Francisco and then helped colleagues around the country.
I was on clinical service that first week of March, when everyone was going into supersonic mode. Our pharmacy team was making sure we had enough drug supply so if we got a surge, we could handle it. That included ordering everything we could think of that might work and listing certain drugs as requiring approval by a member of the infectious disease team.
For example, when the news about hydroxychloroquine first came out, it was killing the coronavirus in the lab. That’s all we knew at that point, but based on a ton of information being pushed out through open-access websites, it seemed like that was going to be what worked, so we bought as much as we could.
On the outside, getting an investigational drug to a patient looks seamless, but on the back end, the amount of work and the number of people who ‘touch’ the patient or the drug is actually huge.”
But the problem was, the information was not peer-reviewed and not based on controlled studies – it was either anecdotal evidence or the methodology wasn’t entirely clear or vetted. As more human data came out, the results were less convincing – there were issues with efficacy and toxicity. It was really a good lesson, showing how results in the lab don’t always translate to people.
On Wednesday night of that week, I woke up at 3:30 in the morning. My brain was on overload at that point, and I thought, “Oh, gosh, I have to check my email.” I picked up my phone, and one of the infectious disease docs had email-blasted the team saying a patient with COVID-19 was coming in, and we needed to get an emergency drug – a new antiviral that was showing promise in early clinical trials but was not yet FDA-approved. She said, “Who’s on? Can we do this?”
I was the one on. The patient was going to be transferred first thing in the morning. Of course, by then I couldn’t sleep, so I thought, “OK, I have a few hours to work on it.” I sat in bed and emailed the doctor a list of the lab tests we needed for an emergency-drug request.
Peter Chin-Hong was the infectious disease (ID) physician who would be on in the morning. After he saw the patient, he sent me the lab results, and I entered everything into the drug company’s emergency-drug request web form. The criteria for emergency use kept changing, but I submitted the request anyway.
I don’t know why I thought it’d be like Amazon, that you’d get an instant confirmation email, but we heard nothing. We were just waiting – we didn’t know if the website worked or if we’d gotten through. It turned out we hadn’t heard because so many people were trying to get through.

Half an hour later, I finally got a response back that the patient was approved. That set the chain of events in motion: The ID consult team got emergency approval from our institutional review board, and the attending physician called the FDA to get emergency approval for single-patient use. Our investigational drug pharmacists had to figure out all the procedures to give this drug safely, since it’s so new. It was a real team effort. On the outside, getting an investigational drug to a patient looks seamless, but on the back end, the amount of work and the number of people who “touch” the patient or the drug is actually huge.
On the national listserv for infectious disease pharmacists, everyone was asking the same question: “Did anyone get through?” I emailed, “Yes, here’s what you do,” and Peter tweeted that at the same time. Then we both got flooded with emails and calls. “Who did you talk to?” “What did you put in this field on the form?” “Can you clarify?” Hospitals across the country were struggling with the same issue.
Peter ended up making a step-by-step diagram of our process over a 72-hour period – what documentation we did, when we called the FDA, when we got the drug. We posted it so other hospitals could figure out the process. That’s how crazy it was – the information was changing literally day by day; everyone just had to be nimble and react to it.
A month later, a colleague texted me that the patient we got the drug for – the one that woke me up at 3:30 in the morning – made it out of the ICU. I was so excited because we all worked so hard to get the drug, and he made it out. As a community at UCSF, everyone gave 500%. It’s a coordinated effort and everybody’s just all in, all the time.
More from this Series
A skilled ventilator operator, respiratory therapist Max Rausch helps keep the sickest patients breathing.