In 1963, Jack Levin, MD, was studying platelets, tiny cells that help human blood coagulate, when his professor suggested he take a look at the horseshoe crab – an ocean-dwelling invertebrate that looks more like a Bronze Age battle helmet than an animal. Levin, then a 30-year-old research fellow at Johns Hopkins University School of Medicine, had never heard of the creature before, but he soon learned that its blood contains a fascinating kind of self-propelled cell found only in invertebrates, known as an amebocyte. He was hooked.
Levin’s curiosity paid off. Over the next 10 years, he helped develop a test that uses amebocytes from horseshoe crabs to check intravenous fluids, injectable drugs, and medical implants for contamination by certain bacterial toxins – a critical measure for preventing adverse reactions. Today, the test is used an estimated 17 million times a year, including most recently to screen components of potential coronavirus vaccines. Back in the ’60s, however, no one could have foreseen this serendipitous discovery. “At the time, it was very unfashionable for MDs in a clinical setting to study nonmammals,” says Levin, now a professor of medicine and of laboratory medicine at UCSF. “There was some muttering that I was off studying a crab,” he recalls, rather than a more familiar animal model like a mouse.
But in recent decades, it’s become more common for medical scientists to learn from organisms off the beaten path. Ferrets, for instance, whose lungs are surprisingly similar to humans’, have helped steer researchers toward potential treatments for cystic fibrosis. Baboons’ blood pressure shoots up when they experience social strife, so studying them has helped scientists understand human hypertension. Even now, as the world battles a novel coronavirus, UCSF scientists are using antibodies inspired by ones discovered in llamas nearly 30 years ago to develop a powerful antiviral nasal spray that could potentially stave off coronavirus infection.
Animals have an astounding array of adaptations that help them forage, fly, wriggle, hunt, sing, multiply, and fight their enemies. Studying them can give researchers crucial insights into how our bodies work. To that end, researchers at UCSF are finding creative ways to learn from a wide variety of fascinating species. From the microscopic nematode worm to the feisty Komodo dragon, a plethora of remarkable organisms are helping scientists understand how we age, how genes function, and how to treat human disease.

Photo: Joel Sartore / National Geographic Photo Ark
The secret to longevity might lie in the
Nothing about a naked mole rat looks normal. The burrowing creatures are mammals, but they’re hairless except for whiskers on their faces, tails, and toes. Their squinting eyes are so small that they’re practically useless. They have buck teeth, no external ears, and skin like a potato that’s been left out for too long.
Nor do the rodents’ lifestyles conform to expectations. They live in colonies with a single queen, like ants or honeybees. They can live for up to three decades – 10 times longer than mice – and almost never succumb to cancer or other diseases of aging. And most interestingly to UCSF’s Diana Laird, PhD, female naked mole rats don’t undergo menopause, so they can keep producing offspring well into their golden years.
A UCSF associate professor of obstetrics, gynecology, and reproductive sciences, Laird studies the development and function of sex cells. She thinks naked mole rats’ reproductive capacity and their bizarrely long life span might be linked. In human females, aging-related diseases seem to ramp up dramatically after menopause. Are the ovaries the body’s canary in the coal mine – the first of many systems to deteriorate – or does their activity actually protect us from disease?
To find out, Laird is collaborating with scientists at the University of Toronto. They send her tissue samples from naked mole rats, which she grows into stem cells that might be coaxed into becoming ovarian cells further down the line. Transplanting the stem cells or ovaries into mice will let her test whether mole rats’ hardy reproductive cells extend the lives of their less-wrinkly relatives – and whether drugs to boost ovarian function could keep humans healthier for longer. “By studying extreme examples in biology,” says Laird, “we can get insights into fundamental principles that we couldn’t otherwise see.”
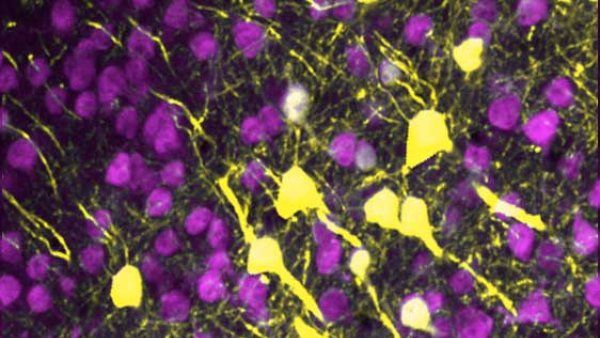
Photo: Willsey Lab, UCSF
A surprising source for untangling autism: the
When Helen Willsey, PhD, looks up from her lab bench, she sees frogs crawling up the walls. The larger-than-life plastic models are surrounded by frog photos, microscope images of frog brains, and a flag featuring a cartoon frog on which someone has taped a black handlebar mustache. Willsey is a postdoctoral researcher in the neurogenetics lab of Matthew State, MD, PhD, the Oberndorf Professor and chair of psychiatry at the UCSF Weill Institute for Neurosciences. She works with a pudgy, speckled creature called the clawed frog to investigate how genes associated with autism affect the development of the brain.
Clawed frogs and humans have some obvious differences. People cannot, for example, survive a drought by burying themselves in mud for a year. But the clawed frog is a popular model for neurological study because their brains develop in similar stages to ours, explains Willsey. Frogs also breed quickly and enthusiastically, laying up to 4,000 embryos in an afternoon, so acquiring research specimens is easy.
Those embryos grow in a more orderly fashion than the embryos of most other organisms. After a fertilized egg divides into two identical cells, one cell will develop into the left half of a frog’s body, and the other cell will grow into the right half. Disabling a gene in just one cell at this stage lets Willsey raise a tadpole that expresses the gene in one half of its body but not the other. Comparing the two halves of the brain under a microscope reveals the effect of that gene on the brain’s development.
Video: Walter Zarnowitz
Willsey has found that several autism-associated genes change the size of the forebrain, which is involved in social interaction and higher thinking. But those differences would have been impossible to detect if Willsey had compared two different frogs, whose sizes vary naturally. “We can pick it up because we have this half-and-half animal,” she says. Translating such research into therapies for humans will be a long and complicated task, but promising leaps in the last few years make Willsey hopeful that the work will pay off.

Photo: Julius Lab, UCSF
Decoding pain through venomous creatures, including the
If you had to incur the wrath of some venomous animal, a black rock scorpion wouldn’t be the worst option. The defensive stings of these 2-inch-long arachnids are so relatively harmless to humans that Australians often keep them as pets. Nonetheless, technicians take care when they extract the black rock scorpion’s venom for scientific research. They hold its pointed tail over a test tube with tweezers, touch an electrode to its body to stimulate its venom gland, and collect the few drops of toxic liquid that trickle out.
David Julius, PhD, the Herzstein Professor and chair of UCSF’s Department of Physiology, keeps vials of the venom in a minus-112-degree freezer. They’re part of a library of venoms he’s assembled from hundreds of stinging and biting animals ranging in dangerousness from the black rock scorpion to the southern man-killer scorpion, which causes more human deaths in its home region of North Africa than any other animal species. Julius is an avid collector of these natural toxins, regardless of whether they’re life-threatening to humans. He wants to know what their neurological effects can teach him about the origins of human pain.
Recently, the black rock scorpion led to one of the lab’s biggest breakthroughs. Though the black rock’s strike feels no worse than a bee sting, tests in mouse cells revealed that its venom attacks the same parts of neurons as spicy mustard, car exhaust, fresh-chopped onions, and wildfire smoke do. However, the black rock’s venom latches onto neurons in a different way than those other chemical irritants do – a way that researchers had not observed before. The black rock scorpion thereby revealed a previously unknown mechanism by which we can feel pain.
By tracing these pathways using various animal venoms, Julius’s team is learning which cells and molecules create pain sensations in the body. Understanding the different ways pain arises is the first step toward finding more effective ways to treat it, Julius says. The next move is up to the pharmaceutical companies, he adds – but “my lab and others in the field are laying the groundwork for drugs that could help people with chronic pain.”

Photo: Joel Sartore / National Geographic Photo Ark
In hopes of regrowing human teeth, scientists look to the
The insides of our mouths are arenas of destruction. When we chew a piece of crusty bread or anxiously clench our jaws at night, our molars grind together with up to 300 pounds of force. Over time, this friction gradually wears away at the rock-hard enamel that coats our teeth. The United States’ multibillion dollar dental industry exists in large part to counteract that process.
Rodents like the prairie vole, on the other hand, have teeth that are “hypselodont” – a mouthful of a descriptor that means their tooth enamel and other dental tissues keep growing throughout the diminutive animals’ lives. Their teeth can achieve this regeneration thanks to stem cells that hide out beneath the gum line. These cells continually divide and grow into new tooth material, which seeps out like magma from a slowly oozing volcano.
Ophir Klein, MD, PhD, a professor of orofacial sciences and pediatrics in the UCSF schools of Dentistry and Medicine, studies rodents like the prairie vole to help decode this process. Unlike mice, whose front teeth are their only teeth that rebuild themselves, the prairie vole’s molars are ever-growing, too. By comparing the genetics and development of species with different numbers of regenerating teeth, Klein seeks to determine how dental stem cells decide whether to stop making tooth material during an animal’s development or to remain active into its adulthood.
Eventually, the work could lead to stem cell treatments that regenerate damaged human teeth, eliminating the need for bridges and fillings, says Klein, who holds the Hillblom and Epstein Professorships. Although the path there isn’t entirely clear yet, “history has shown us that when we explore all the interesting things that nature has figured out how to do, in the long run it’s productive,” he says.

Photo: Joel Sartore / National Geographic Photo Ark
Solving speech problems by studying the
Michael Brainard, PhD, has a music studio in his laboratory. It’s divided into 20 compartments where different singers can perform. But each sound booth is only about the size of a standard kitchen range. That’s because Brainard, a UCSF professor of physiology and psychiatry, studies the crooning of pint-size male songbirds, including the Bengalese finch.
Songbirds learn vocalizations in much the same way human babies do. They copy the songs of adult birds that raise them, show evidence of regional “dialects,” and need to hear themselves practicing their trills and twitters in order to improve. Like human speech, birdsong also requires fine control of a complex system of muscles. Air has to squeeze out of the lungs, ruffle the vocal cords, resonate through the mouth, and roll off the tongue in just the right ways to produce a particular set of sounds.
By recording finches’ brain activity as they learn songs from computerized teachers, Brainard’s lab has pieced together the neural networks that coordinate the cognitive and motor skills involved in vocal learning. They’ve found that the basal ganglia, a group of nerve clusters buried deep within the brain, are integral to the process. When the scientists inactivate the basal ganglia, the birds’ practice songs become more monotonous, says Brainard. “It looks like this part of the brain is introducing variability – constantly doing things a little differently and then discovering ‘Okay, that sounded really good. I’ll do it that way again.’”
Video: Walter Zarnowitz
Another thing finches and humans have in common is that vocal learning is relatively easy when individuals are young and impressionable, but it becomes much more difficult after early adolescence. While humans have until their tween years to assimilate a new accent, for example, in finches this flexible period lasts for only a few months. Deciphering the details of how this shift happens is the first step toward potentially enhancing adult learning, which could aid in treating strokes and other conditions that affect speech and movement. “If we could understand what limits learning in adult songbirds,” says Brainard, “that would be a starting point for understanding what goes wrong in the more complex setting of human disease.”
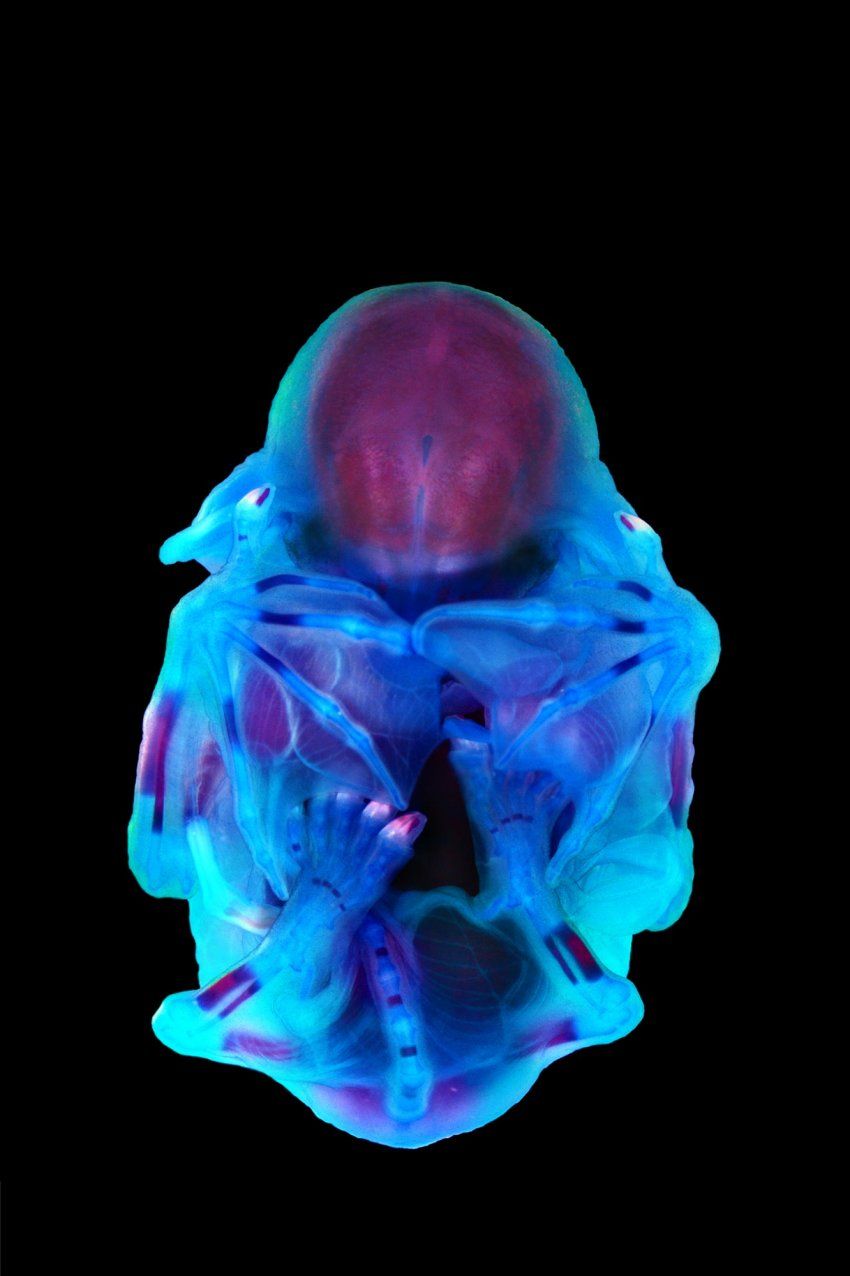
Photo: Mandy Mason, University of Cape Town
Learning about birth defects from the
In 2012, at a scientific conference in Canada, geneticist Nadav Ahituv, PhD, sat down to eat a salmon salad. He struck up a conversation with Nicola Illing, PhD, an evolutionary biologist at the University of Cape Town, who happened to be sitting nearby. Illing mentioned that she’d started working with long-fingered bats, which gather to breed in South Africa. Ahituv immediately suggested they collaborate.
Ahituv, a professor of bioengineering and therapeutic sciences in the UCSF School of Pharmacy, studies human congenital limb malformations, such as webbed toes or extra fingers. Talking with Illing, he realized that bats, whose wings stretch across their spindly finger bones, could help him investigate how such malformations arise. Although similar genes encode the plans for both bats’ forelimbs and their hind ones, the rear set develops into small feet instead of splayed-out wings. Ahituv wanted to know which molecular switches tell cells in each location to interpret their instructions differently.
His collaboration with Illing works like this: Illing collects embryos from pregnant bats, determines how far along each embryo is in its development, and then ships some of the embryos on ice to Ahituv’s lab. There, scientists search the partly formed limbs for RNA molecules – tiny transcripts that cells make in order to carry out the instructions of a particular gene – and the DNA sequences that act as switches to tell the transcription process to start. By comparing the RNA and switches in the forelimbs and hind limbs at different developmental stages, the scientists can identify which genes trigger bones to lengthen, muscle fibers to knit together, or webbing between digits to expand or retract.
Ahituv’s lab has so far identified more than 3,000 genes and thousands of switches that could be involved in bat wing formation. Other vertebrates, including humans, have many of the same genes, explains Ahituv. Therefore, decoding the limb-development process in bats could help scientists create prenatal genetic screenings that warn human parents-to-be of potential developmental problems in a fetus. Beyond diagnostics, he adds, knowing the causes of limb malformations “might enable you to treat them.”
What drives our appetites? Ask a
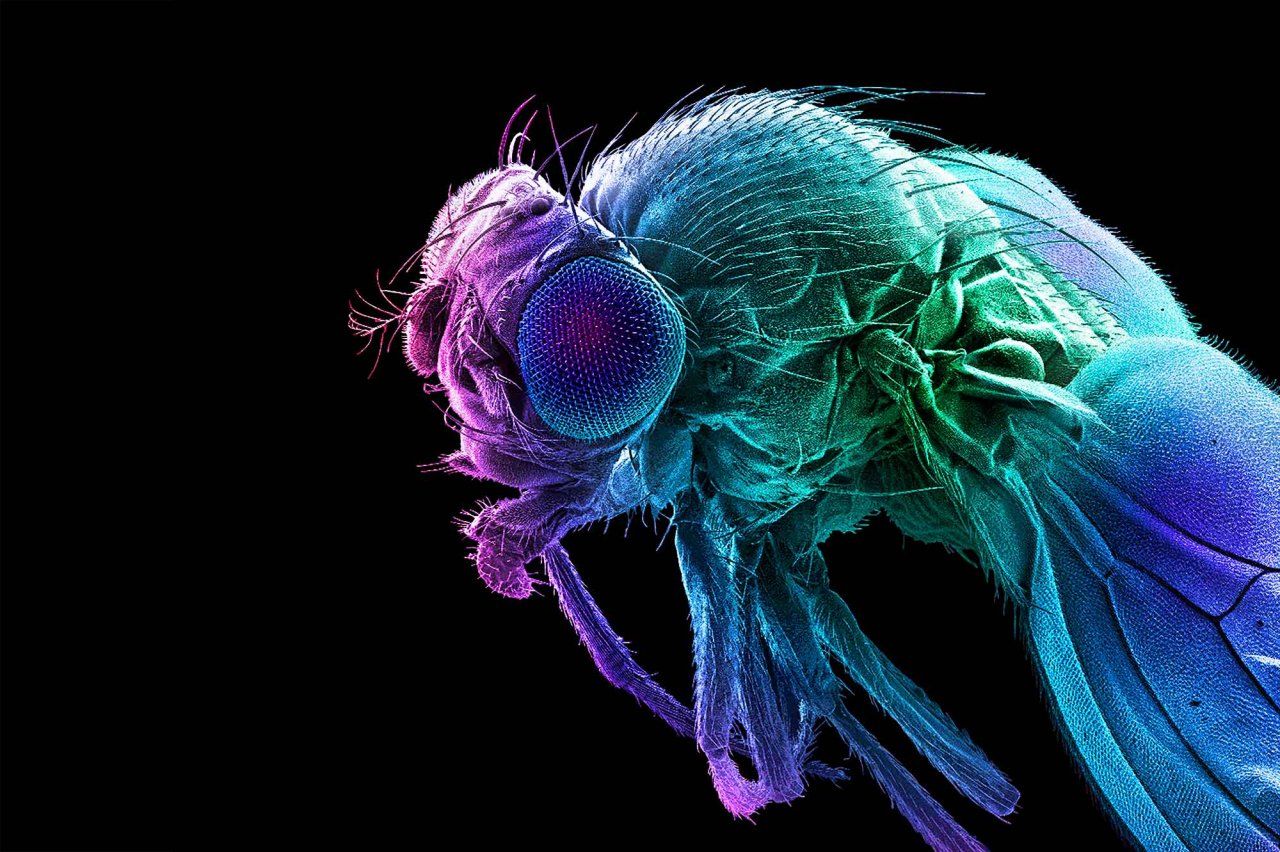
Qili Liu, PhD, usually finds the taste of eggs unbearable. But in 2015, when she was pregnant with her first child, she found herself craving them constantly. At the time, Liu was a postdoctoral researcher at Johns Hopkins University, studying what makes tiny fruit flies hungry. She wondered if her uncharacteristic urges signaled her body’s need for a specific nutrient: protein.
That question was still on Liu’s mind when she joined the UCSF faculty in 2019 and started her own lab as an assistant professor of anatomy. Though scientists understand fairly well how the body regulates its total energy intake, she explains, “we actually don’t know much about how we choose specific types of food.” Her lab investigates how changes in fruit flies’ circumstances make them seek out particular nutrients such as protein, fat, or sugar. How, for instance, does a fly’s body actually detect a nutrient deficiency? Which neurons in the brain then translate that internal reading into a specific craving?
These questions aren’t relevant only to fruit flies, of course. Untangling how particular nutrients affect hunger and meal choices can help scientists understand some all-too-human problems such as overeating and obesity. But studying tiny flies instead of people has some distinct advantages. For one, a fly’s brain contains only about 100,000 neurons – compared to a human brain’s 100 billion – and scientists have genetic tools that help them identify the ones that fire when the fly behaves in a particular way.
Liu can also breed fruit flies quickly, and with different combinations of genes, to see which genes influence how their brains act while they’re feeding. Such experiments would never be possible – or ethical – with human subjects. But Liu’s early work suggests that the comparison will be advantageous: Among other similarities, she’s found that pregnant female flies eat more protein. “They have a striking parallel to humans,” she says. “I’m not sure every pregnant woman craves eggs, but in my case, it was a hint that drove me to investigate the protein appetite.”
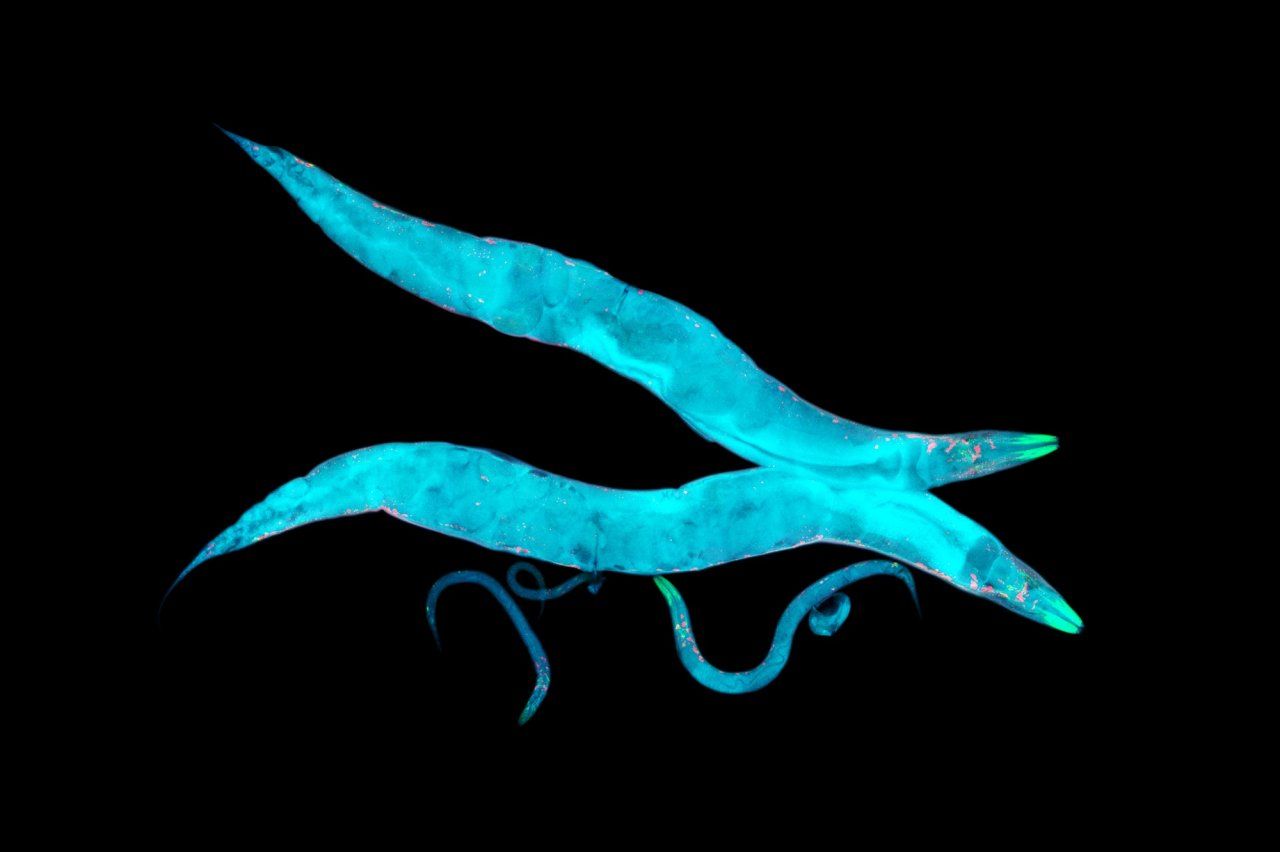
Photo: Heiti Paves
Unraveling Alzheimer’s with help from a lowly worm, the
It takes a certain type of person to find a nematode adorable. Caenorhabditis elegans is a millimeter long, shaped like a squirt of toothpaste, and usually content to make its life in a malodorous compost heap. But Aimee Kao, MD, PhD, finds the worms quite charming as they forage for bacteria to eat in a petri dish. “You get the sense that they’re intentional – they have loads of personality,” she says.
Kao, an associate professor of neurology at the UCSF Weill Institute for Neurosciences, is one of thousands of scientists worldwide using C. elegans for medical research. The nematodes help her study neurodegenerative diseases such as Alzheimer’s and dementia. Scientists know that a buildup in the brain of certain proteins, known as beta-amyloid and tau, contributes to these conditions in humans. What they don’t know is why the proteins build up in the first place, or how exactly their presence causes the brain’s function to go awry.
C. elegans’s genome is roughly similar to that of humans. It can easily be engineered to pump out beta-amyloid and tau proteins, allowing Kao’s team to investigate their effects and search for therapies. Unlike humans, however, nematodes live for only about two weeks – meaning they age about 1,700 times faster than we do, revealing the proteins’ impact on the brain in a reasonable enough time frame for a laboratory experiment. As an extra benefit, the worms are transparent. That allows Kao to peer through a microscope and see nematode neurons at work while her subjects are still alive.
Video: Walter Zarnowitz
Kao’s lab breeds nematodes with different combinations of genes that are thought to modulate brain function. Within weeks, her team can see how each new combination affects levels of beta-amyloid and tau or the performance of the cellular cleanup systems that normally break down these proteins. By helping Kao tease out specific genes that influence neurodegenerative diseases, tiny nematodes could eventually inspire new tests and treatments. “They’re super cute little things,” Kao says, “and we just appreciate what they’ve taught us about human health, biology, and disease.”

Photo: Joel Sartore / National Geographic Photo Ark
This big, cranky lizard offers clues to healthier hearts. Meet the
In 1986, President Ronald Reagan traveled to Bali to meet with President Suharto of Indonesia. As a parting gift, Suharto promised the United States two Komodo dragons – the 10-foot, 150-pound, famously cantankerous lizards that are native only to the Indonesian islands. The scaly pair, named Sobat and Friendty, arrived at the National Zoo in Washington, D.C., and in 1992 welcomed 13 healthy babies, making them the first Komodo dragons to breed successfully in captivity. Decades later, blood samples from one of those offspring – a sun-loving hulk named Slasher – landed in the lab of Benoit Bruneau, PhD.
As director of the Gladstone Institute of Cardiovascular Disease and a professor of pediatrics at UCSF, Bruneau studies the development of the human heart. He’s particularly curious about how glitches in the process lead to congenital heart defects. Komodo dragons, at a glance, seem extremely different from humans: Their long, curved teeth have knife-like ridges, and their painful bite can inject enough venom to kill a water buffalo. But stalking and striking such supersized prey requires extraordinary cardiovascular endurance, and that’s where Bruneau’s interest in the creatures comes in.
“Komodo dragons can ramp up their metabolism to near-mammalian levels,” Bruneau explains. That means that every cell of the animal’s heart is working overtime when it hunts. Bruneau – in collaboration with his UCSF colleagues Katherine Pollard, PhD, a professor of epidemiology and biostatistics, and Pui-Yan Kwok, MD, PhD, the Bachrach Distinguished Professor of Cardiovascular Genetics – learned more about these monumental bursts of effort by sequencing the genomes of three dragons, including Slasher. Their labs compared the results to the genomes of other lizards to identify dozens of genes that seem to be related to the extraordinary function of the Komodo dragon’s heart. Eventually, the work could help scientists identify and screen for genes responsible for certain heart defects in humans. “We look back in evolution to be able to look forward in understanding disease,” Bruneau says.